
Think of the HPLC Column as the Heart of
the HPLC System. It's the component where the actual separation of the
components in your mixture takes place.
Simple Analogy: Imagine a crowd of people (your sample mixture) running through a
forest (the column). People who stop to look at trees (interact with the
column) will move more slowly. People who run straight through (don't interact)
will move faster. Because each person has a different level of interest in
trees, they will exit the forest at different times, thus becoming separated.
The HPLC column is that forest, designed to create differences in the travel
time for each compound.
Technical Definition: An HPLC column is a stainless-steel tube tightly packed with very small, porous particles (the stationary phase). These particles have a specific surface chemistry that interacts with the chemical compounds (analytes) in your sample as they are pushed through by a liquid (the mobile phase).
Key Components of an HPLC Column:
- Column Hardware: Typically,
a stainless-steel tube capable of withstanding high pressures (up to 1000
bar or more in UHPLC).
- Stationary Phase: The
packing material inside the tube. This is the most critical part that
defines the column's selectivity. It consists of:
- Base Particle: Usually silica or
polymer beads, typically 1.7 to 5 micrometres in diameter. Smaller
particles give higher efficiency but require higher pressure.
- Surface Chemistry: A layer of molecules chemically bonded to the base particle. This layer determines how the column interacts with your compounds. Common types include C18, C8, Phenyl, etc.
HPLC Column Selection Guide
Selecting the right column is the most critical
step in developing an HPLC method. There is no single "best" column;
the choice depends entirely on your sample.
Here is a step-by-step guide to selecting an HPLC column, summarized in the flowchart below:
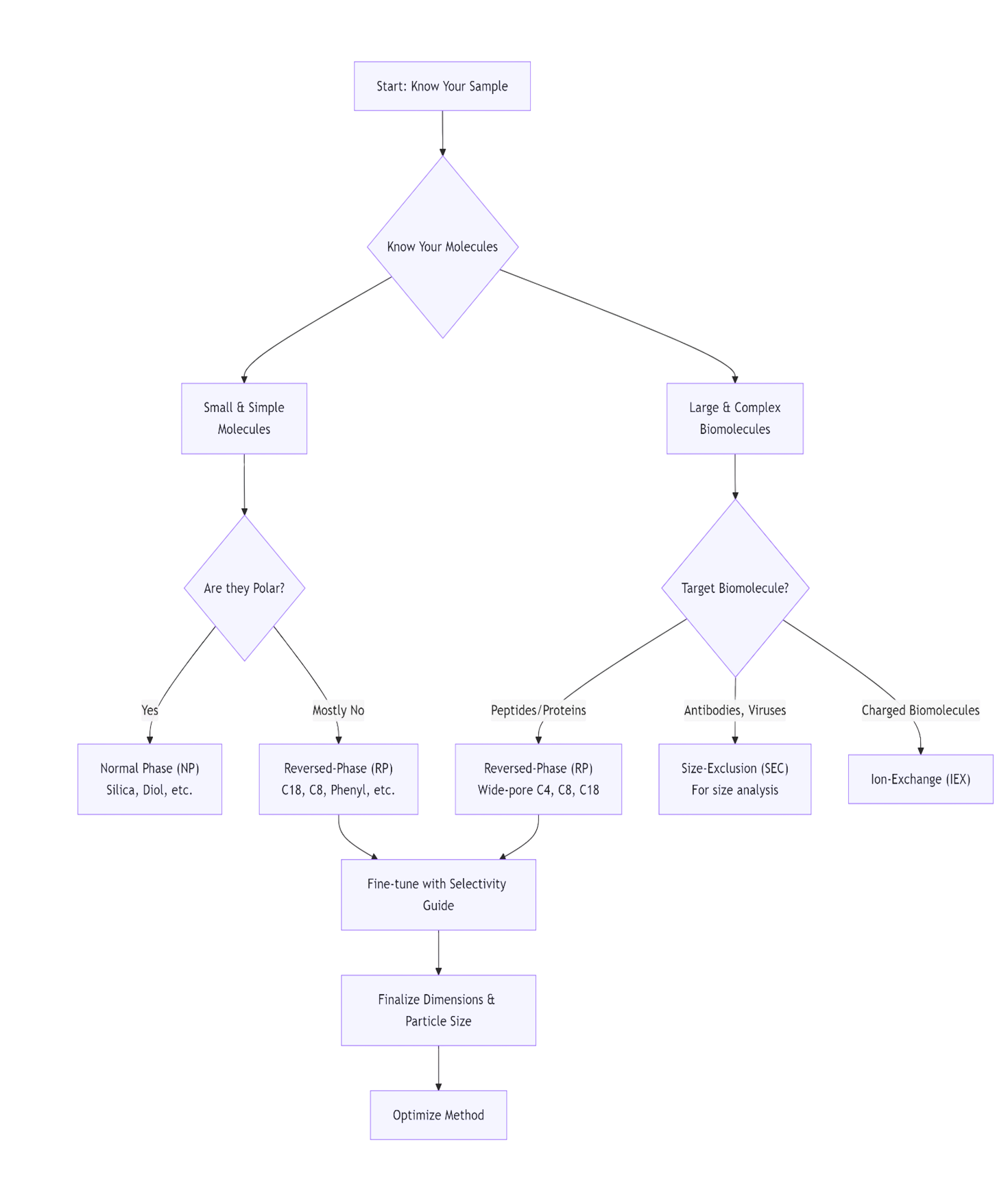
Now, let's walk through each of these steps in detail.
Step 1: Understand Your Sample and Goal
This is the "know your enemy" step.
- What are the compounds? Their
chemical structures, molecular weights, and functional groups.
- What is their polarity? Are
they hydrophobic (non-polar) or hydrophilic (polar)?
- Are they ionic or ionizable? Do
they have acidic/basic groups?
- What is the sample matrix? (e.g.,
plasma, soil, food extract)
- What is the goal? Qualitative
analysis, quantitative analysis, or preparative purification?
Step 2: Choose the Separation Mode (The Big Choice)
This decision is primarily based on the polarity and size of
your analytes, relative to the sample matrix.
- Reversed-Phase (RP)
- Mechanism: Partitioning between
a non-polar stationary phase and a polar mobile phase.
- Stationary Phase: Non-polar (e.g.,
C18, C8, C4, Phenyl).
- Mobile Phase: Polar (e.g.,
Water, Methanol, Acetonitrile).
- Use Case: ~80% of all HPLC
applications. Ideal for non-polar to moderately polar,
water-soluble molecules. Excellent for pharmaceuticals, food analysis,
environmental samples.
- Elution Order: Most polar
compounds elute first, most non-polar last.
- Normal-Phase (NP)
- Mechanism: Partitioning between
a polar stationary phase and a non-polar mobile phase.
- Stationary Phase: Polar (e.g.,
Silica, Cyano, Diol, Amino).
- Mobile Phase: Non-polar (e.g.,
Hexane, Chloroform, Ethyl Acetate).
- Use Case: ~10% of
applications. Ideal for highly polar, water-insoluble
compounds, isomers, and chiral separations.
- Elution Order: Most non-polar
compounds elute first, most polar last.
- Ion-Exchange (IEX)
- Mechanism: Interaction between
charged analytes and oppositely charged groups on the stationary phase.
- Stationary Phase: Charged
groups (e.g., -SO₃⁻ for Cation Exchange, -N(CH₃)₃⁺ for Anion Exchange).
- Use Case: Separation of ions,
proteins, nucleotides, sugars.
- Size-Exclusion (SEC)
- Mechanism: Sieving of molecules
based on their hydrodynamic size (not molecular weight directly).
- Stationary Phase: Porous
particles with a specific pore size range.
- Use Case: Separating proteins,
polymers, and large biomolecules. Also, for desalting.
- Elution Order: Largest molecules
elute first, smallest last.
Step 3: For Reversed-Phase (The Most Common
Scenario), Follow the Selectivity Guide
Since RP-HPLC is so dominant, here’s how to choose within this category:
|
Stationary Phase |
Key Characteristics |
Best For |
|
C18 (ODS, L1) |
Gold Standard. Highest hydrophobicity, greatest retention. |
General purpose, non-polar to medium-polar
compounds. |
|
C8 (L7) |
Slightly less hydrophobic than C18. Similar
selectivity. |
Good for molecules that are too retained on
C18. Often used for peptides and proteins. |
|
Phenyl (L11) |
π-π interactions with aromatic rings.
Different selectivity than alkyl chains. |
Aromatic compounds, isomers. |
|
Phenyl-Hexyl |
Combines π-π interactions of phenyl with the
hydrophobicity of a hexyl chain. |
A great alternative to C18 for different
selectivity. |
|
Cyano (CN, L10) |
Weak hydrophobicity, slightly polar. |
Can be used in both Reversed-Phase and
Normal-Phase modes. Good for polar analytes. |
|
Pentafluorophenyl (PFP) |
Strong π-π interactions, dipole-dipole
interactions, and shape selectivity. |
Excellent for separating complex mixtures of
isomers (positional, structural). |
General Rule of Thumb: Start method development with a C18 column. If the
separation is poor, switch to a column with a different selectivity (e.g.,
Phenyl or PFP).
Step 4: Choose Column Dimensions and Particle Size
This affects efficiency, speed, and pressure.
- Length (L):
- 50-150 mm: Standard for most
analytical applications. Good balance of speed and resolution.
- >150 mm (e.g., 250 mm): Higher
resolution for complex mixtures but longer run times and higher pressure.
- <50 mm (e.g., 30 mm): Fast
analysis for simple mixtures, lower resolution.
- Internal Diameter (ID):
- 4.6 mm: Standard analytical
column.
- 2.1 mm: For LC-MS (better
compatibility, lower flow rates).
- 1.0 mm and below: Micro/nano-LC
for limited samples or specialized MS.
- Particle Size (dp):
- 3 µm to 5 µm: Standard for HPLC.
Good efficiency and pressure.
- <2 µm (e.g., 1.7-1.8 µm): For UHPLC.
Higher efficiency and faster analysis, but requires instruments that can
handle very high pressure.
Summary & Quick-Start Guide
- Unknown Sample? Start with a Reversed-Phase
C18 column.
- Common Starting Dimensions: 150 mm or 100 mm long, 4.6 mm
ID, 5 µm or 3 µm particle size.
- If compounds are too retained (elute too late), use a weaker mobile
phase or a less retentive column (e.g., C8 or Cyano).
- If compounds elute too quickly (no retention), use a stronger
mobile phase or a more retentive column (e.g., C18).
- If you have poor resolution between peaks, try a column with a
different selectivity (e.g., Phenyl or PFP) or adjust the mobile phase
pH/organic modifier.
- Always check the literature! Someone
has probably analyzed something similar to your sample. Start with their
conditions.
By following this logical process, you can systematically narrow down the vast world of HPLC columns to the few that are most likely to work for your specific application.

Leave a comment